
Protons are the permanent core particles of an atom. Therefore, the number of negatively charged electrons orbiting in its orbit is equal to the number of positively charged protons in the nucleus.Ītomic number (Z) = Number of charges in the nucleus (p) How many protons does a helium atom have? That is, the atomic number is the total number of protons. We know that protons are located in the nucleus of an atom as a positive charge.
#OTIXO HONEST REVOEWS SERIAL NUMBER#
This number is equal to the serial number of the periodic table. The atomic number of the element is expressed by ‘Z’. Thus, the number of positive charges present in the nucleus of an element is called the atomic number of that element. He called that number the order of the atoms. The results of his experiments show that each element has a unique integer equal to the number of positive charges in the nucleus of that element. Scientist Henry Gwynn Jefferies Mosle examined the X-ray spectrum of various elements in 1913-to 1914.
#OTIXO HONEST REVOEWS HOW TO#
How to easily find the number of electrons, protons and neutrons in a helium atom? Electrons revolve around the nucleus in a specific orbit. The only exception is hydrogen, which has only protons in its nucleus but no neutrons. Experiments by various scientists have shown that the nucleus of an atom contains protons and neutrons. One is the nucleus and the other is the orbit. Also, neutrino, antineutrino, positron, and mason are located in an atom as temporary particles.Ītoms can usually be divided into two parts. Numerous permanent and temporary particles exist in the atom.Įlectrons, protons, and neutrons are located in the atom as permanent particles. However, it has been possible to detect atoms by increasing the vision of a very powerful electron microscope by two million times. So, if 1000 crore atoms of hydrogen are arranged side by side, it will be 1 meter long. The diameter of an atom of hydrogen is 0.1nm (1.0nm = 10 -9m). Atoms are so small particles that they cannot be seen even under a powerful microscope. Where are the electrons, protons and neutrons located in an atom?Īn atom is the smallest particle of an element that has no independent existence but is directly involved in chemical reactions as the smallest unit.

Hopefully, after reading this article you will know the details about this topic. This article discussed in detail how to easily determine the number of protons, neutrons, and electrons in a helium atom.Īlso discussed is the position of electrons, protons, and neutrons in an atom, the number of atomic masses, and the isotopes of helium. The helium element has two stable isotopes. The number of neutrons depends on the isotope of the element. Therefore, a helium atom has two neutrons. The difference between the mass number of the helium atom and the number of protons is two. The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons. Therefore, a helium atom has two protons and two electrons. The atomic number of an element is equal to the number of protons and electrons in that element. Helium is the 2nd element of the periodic table so its atomic number is 2. Click ‘Grant access’ to sync the account.The first noble gas of group-18 of the periodic table is helium and its symbol is He. A request will be sent to the cloud service provider and you may need to sign in to confirm. Adding a Display NameĮnter your name and click ‘Authorize’. All popular cloud apps are supported just select your service and continue. After the signup choose the cloud service that needs to be linked to the Otixo account.
#OTIXO HONEST REVOEWS FOR FREE#
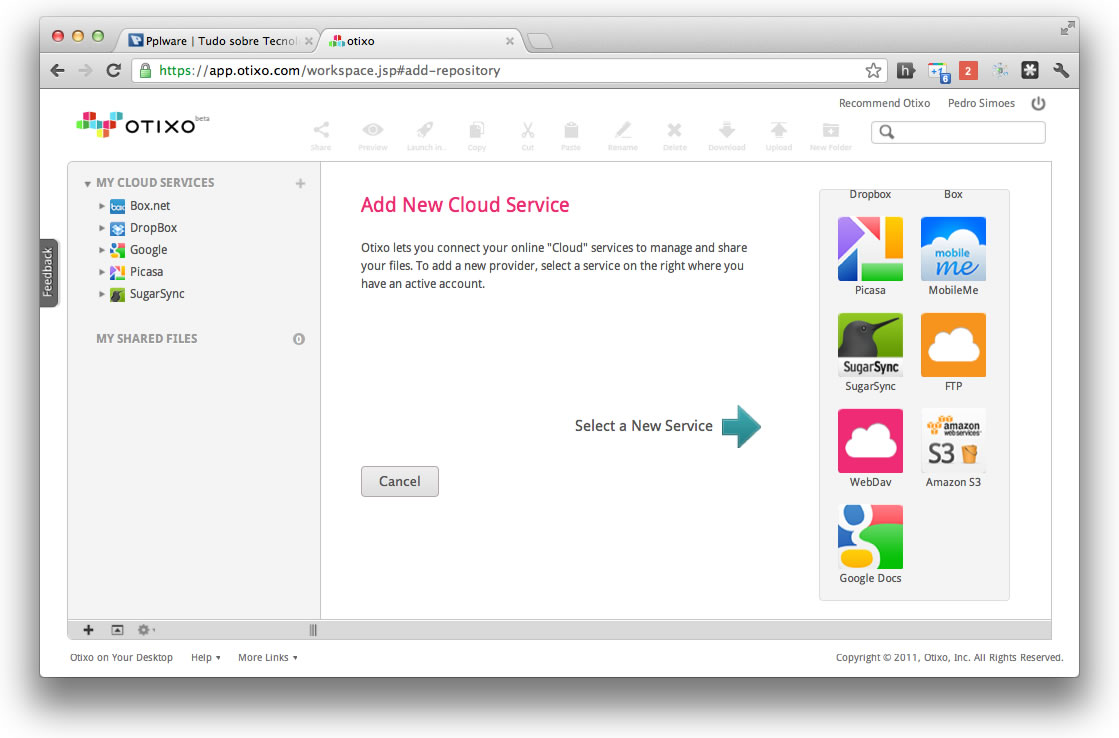
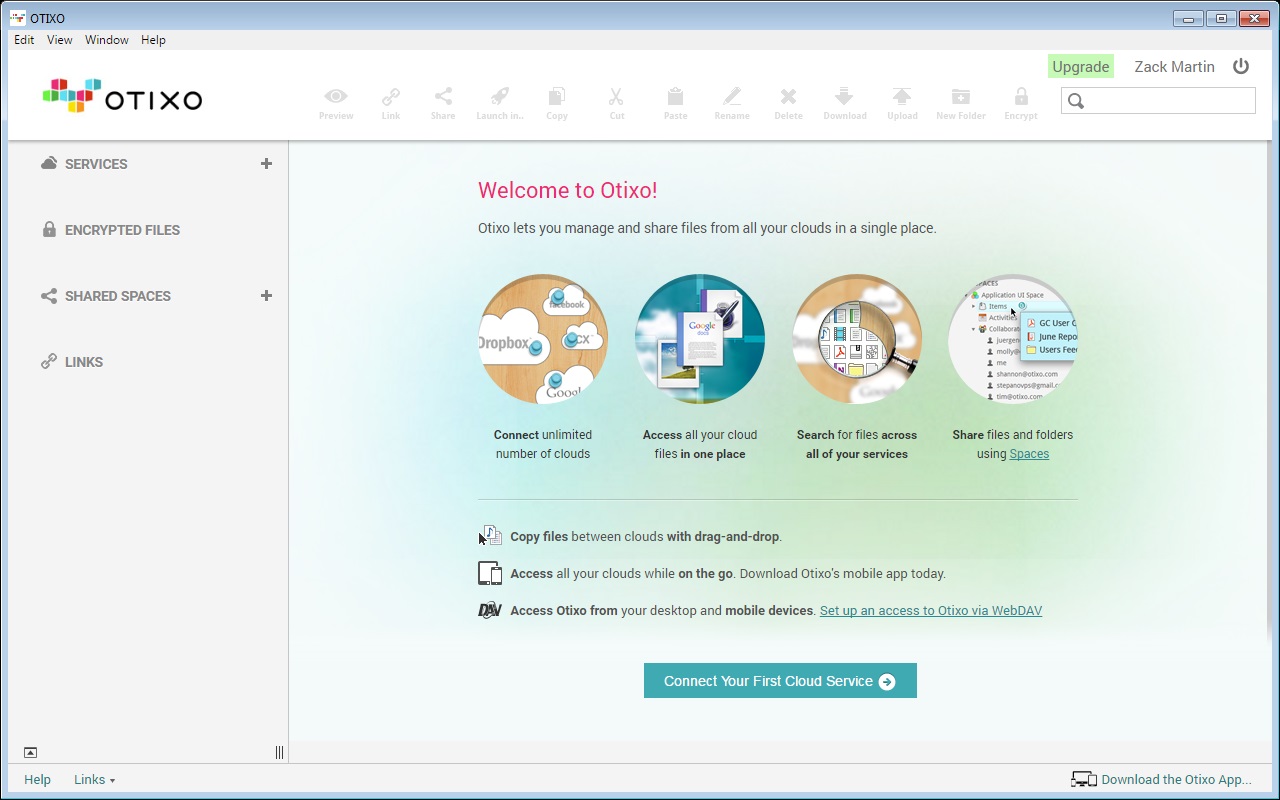
To use the free service go to Otixo and signup for free account. You can get 250 MB of free bandwidth usage for per month or pay $9.99 for Unlimited Bandwidth Usage. With OTIXO web service you can connect services like Dropbox, Box, Google Docs, SugarSync, Picasa, MobileMe, Amazon S3, FTP sites and WebDav drives in one place. But with the help of OTIXO you can access and manage all your online files in one single place. And also it difficult to remember which file are saved in which service. Having several free online storage accounts give you lots of space to store your files, but it very difficult to manage all these files same time.


 0 kommentar(er)
0 kommentar(er)
